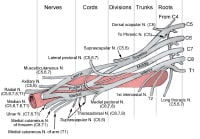
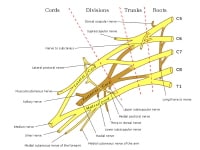
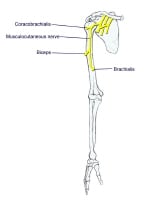
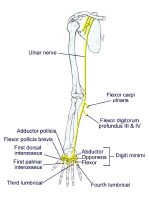
Overview
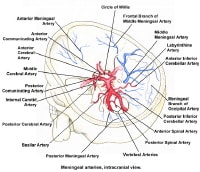
The circle of Willis (circulus arteriosus cerebri) is an anastomotic system of arteries that sits at the base of the brain. The “circle” was named after Thomas Willis by his student Richard Lower. Willis was the author of Cerebri Anatome, a book that described and depicted this vascular ring. Although such a vascular ring had been described earlier, the name Willis has been eponymously propagated.
The circle of Willis encircles the stalk of the pituitary gland and provides important communications between the blood supply of the forebrain and hindbrain (ie, between the internal carotid and vertebrobasilar systems following obliteration of primitive embryonic connections). A complete circle of Willis is present in most individuals, although a well-developed communication between each of its parts is identified in less than one half of the population.
The circle of Willis (circulus arteriosus cerebri) is an anastomotic system of arteries that sits at the base of the brain. The “circle” was named after Thomas Willis by his student Richard Lower. Willis was the author of Cerebri Anatome, a book that described and depicted this vascular ring. Although such a vascular ring had been described earlier, the name Willis has been eponymously propagated.
The circle of Willis encircles the stalk of the pituitary gland and provides important communications between the blood supply of the forebrain and hindbrain (ie, between the internal carotid and vertebrobasilar systems following obliteration of primitive embryonic connections). A complete circle of Willis is present in most individuals, although a well-developed communication between each of its parts is identified in less than one half of the population.
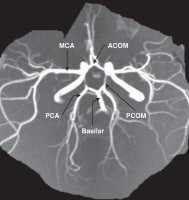
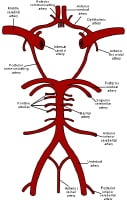
The circle of Willis is formed when the internal carotid artery enters the cranial cavity, bilaterally, and divides into the anterior cerebral artery (ACA) and middle cerebral artery (MCA). The anterior cerebral arteries are then united by an anterior communicating artery (ACOM). These connections form the anterior half (anterior circulation) of the circle of Willis. Posteriorly, the basilar artery, formed by the left and right vertebral arteries, branches into a left and right posterior cerebral artery (PCA), forming the posterior circulation. The posterior cerebral arteries complete the circle of Willis by joining the internal carotid system anteriorly via the posterior communicating arteries (PCOM).
The images below depict the circle of Willis.
Gross Anatomy
Anterior Cerebral Artery
A1 segment and anterior communicating artery
The A1 segment extends from the internal carotid artery (ICA) bifurcation in a medial and superior direction to its junction with the anterior communicating artery (ACOM) within the longitudinal fissure. Branches include the medial lenticulostriate arteries (A1) that supply the anterior hypothalamus, anterior commissure, fornix, striatum, optic chiasm, and optic nerves. ACOM branches include perforators that supply the hypothalamus and optic chiasm.
A2 segment
This portion of the anterior cerebral artery extends from the ACOM artery to its division into the pericallosal and callosomarginal arteries, at the genu of the corpus callosum. Branches include perforators to the frontal lobe, and the recurrent artery of Heubner, which is a large lenticulostriate vessel. This latter vessel supplies the caudate nucleus, internal capsule, and putamen. Other branches of A2 include the orbitofrontal and frontopolar arteries.
A3 segment
These include all branches of the anterior cerebral artery (ACA) distal to the origin of the pericallosal and callosomarginal arteries, but other subdivisions have been used. Many anastomoses occur with distal branches of the middle cerebral artery (MCA) and posterior cerebral artery (PCA).[2] The pericallosal artery travels posteriorly over the corpus callosum and anastomoses with the splenial artery. The callosomarginal artery courses over the cingulate gyrus. A paracentral artery arises from the pericallosal or callosomarginal arteries and supplies the paracentral lobule. This segment terminates by providing parietal arteries to the corpus callosum and precuneus.
Middle Cerebral Artery
Most classification schemes divide the middle cerebral artery (MCA) into 4 segments, including M1 from the ICA to the bifurcation (or trifurcation), M2 from the MCA bifurcation to the circular sulcus of the insula, M3 from the circular sulcus to the superficial aspect of the Sylvian fissure, and M4, which are cortical branches.
M1 segment
Most anatomic studies define the M1 segment as ending where the MCA branches take a right angled turn within the Sylvian fissure; however, the division point of the MCA trunk is considered by most clinicians to be the M1/M2 junction.[3] The MCA most commonly bifurcates but may also trifurcate or quadfurcate.[4] Branches include lenticulostriate arteries that supply the anterior commissure, internal capsule, caudate nucleus, putamen, globus pallidus, and an anterior temporal artery that supplies the anterior temporal lobe.[5]
M2 segment
The M2 segment extends from the main division point of the M1 segment, over the insula within the Sylvian fissure, and terminates at the margin of the insula.
M3 segment
M3 segment
The M3 segment begins at the circular sulcus of the insula and ends at the surface of the Sylvian fissure. This part travels over the surface of the frontal and temporal operculae to reach the external surface of the Sylvian fissure. The M3 and M2 segments give rise to stem arteries from which cortical branches are derived.
M4 segment
M4 segment
The M4 segment begins at the surface of the Sylvian fissure and extends over the surface of the cerebral hemisphere. Its cortical branches supply the frontal, parietal, temporal, and occipital lobes. These branches include orbitofrontal, prefrontal, precentral, central, anterior, and posterior parietal, angular, temporo-occipital, temporal, and temporopolar branches. The MCA branches that form the so-called "candelabra" are the prefrontal, precentral, and central arteries.
Posterior Cerebral Artery
A commonly used subdivision for this vessel includes dividing it into a P1 segment from the basilar artery bifurcation to the junction with the posterior communicating arteries (PCOM) artery, a P2 segment from the PCOM artery to the posterior aspect of the midbrain, a P3 segment from the posterior aspect of the midbrain to the calcarine fissure, and a P4 segment that describes terminal branches of the PCA distal to the anterior aspect of the calcarine fissure.
P1 segment andposterior communicating arteries
The P1 segment supplies perforating branches to the brainstem. These are termed the posterior thalamoperforators to distinguish them from the anterior thalamoperforators, which arise from the PCOM artery. The direct perforators supply the thalamus, brainstem, and internal capsule. Short and long circumflex arteries supply the thalamus and midbrain. A meningeal branch may supply the inferior surface of the tentorium cerebelli.[7]
P2 segment
P2 segment
The P2 segment begins at the PCOM artery junction and travels around the lateral aspect of the midbrain. Direct perforators supply the thalamus, internal capsule, and the optic tract. Branches include the posteromedial choroidal artery that supplies the midbrain, pineal gland, thalamus, and medial geniculate body and the posterolateral choroidal artery that supplies the choroid plexus, thalamus, geniculate bodies, fornix, cerebral peduncle, pineal body, corpus callosum, tegmentum, and temporal occipital cortex. A hippocampal artery may be present. The inferior temporal arteries anastomose with anterior temporal branches of the MCA. The parieto-occipital artery arises as a single trunk from the P2 segment more commonly than from the P3 segment. This artery supplies the posterior parasagittal region, cuneus, precuneus, and lateral occipital gyrus.
P3 segment
P3 segment
The P3 segment extends from the tectum to the anterior aspect of the calcarine fissure. The PCA often divides into its 2 terminal branches, the calcarine and parieto-occipital arteries.
P4 segment
P4 segment
The P4 segment begins at the anterior limit of the calcarine fissure and often includes one of the 2 main terminal branches of the PCA, the calcarine artery. The other main terminal branch of the PCA, the parieto-occipital artery, frequently arises from the P2 or P3 segment. The splenial artery arises from the parieto-occipital artery in most individuals and usually anastomoses with the pericallosal artery.
Basilar Artery
The basilar artery originates at the junction between the left and right vertebral arteries and travels anterior to the brainstem. Branches include the superior cerebellar artery and anterior inferior cerebellar artery (AICA).[9] The superior cerebellar artery (SCA) arises from the basilar artery immediately prior to the basilar bifurcation. The SCA often comes into contact with the trigeminal nerve and is usually the target of surgical microvascular decompression for trigeminal neuralgia.
The artery sends branches to the tectum, vermis, and the medial aspect of the cerebellar hemisphere. AICA travels toward the cerebellopontine angle. The posterior inferior cerebellar artery (PICA) is the largest of the cerebellar arteries and arises from the vertebral artery. It supplies the medulla, cerebellar tonsils and vermis, and inferolateral cerebellar hemisphere.
The artery sends branches to the tectum, vermis, and the medial aspect of the cerebellar hemisphere. AICA travels toward the cerebellopontine angle. The posterior inferior cerebellar artery (PICA) is the largest of the cerebellar arteries and arises from the vertebral artery. It supplies the medulla, cerebellar tonsils and vermis, and inferolateral cerebellar hemisphere.
Natural Variants
Anterior circulation
The anterior cerebral arteries may be united in a single trunk, which runs in the longitudinal fissure, giving branches to both hemispheres. The left and right A1 segments are asymmetric in size in most individuals and may be absent or fenestrated. Rarely, this segment may travel inferior to or through the optic nerve. An accessory ACA may be present, and the A1 segment may arise from the cavernous or contralateral ICA.
Either the right and left anterior cerebral arteries may run as one vessel (azygos) to divide distally, or one may be a branch of the contralateral artery. Other variations of the ACOM include aplasia, fenestration, and duplication.[10] This vessel may be curved, kinked, or tortuous. The artery is rarely absent. One A2 segment may be hypoplastic; thus, the contralateral A2 supplies both hemispheres. A2 may be duplicated. In an azygos ACA, both A1 segments join to form a single A2 segment. Branches to the contralateral hemisphere may be found.
Either the right and left anterior cerebral arteries may run as one vessel (azygos) to divide distally, or one may be a branch of the contralateral artery. Other variations of the ACOM include aplasia, fenestration, and duplication.[10] This vessel may be curved, kinked, or tortuous. The artery is rarely absent. One A2 segment may be hypoplastic; thus, the contralateral A2 supplies both hemispheres. A2 may be duplicated. In an azygos ACA, both A1 segments join to form a single A2 segment. Branches to the contralateral hemisphere may be found.
Posterior circulation
When a fetal PCOM artery is present, the ipsilateral P1 is typically hypoplastic. Variations of the P1 segment include duplication, fenestration, and a bilateral shared origin of the PCA and SCA. A prominent perforating branch may supply portions of both the ipsilateral and contralateral thalamus and potentially the mid brain. The posterior cerebral may course below, rather than over, the oculomotor nerve, or it may be absent and replaced by an accessory contralateral vessel. The posterior cerebral may give rise to the anterior cerebral artery. The posterior cerebral artery may arise from the internal carotid.
The PCOM may be absent, or the branch representing it may fail to join the posterior cerebral. Fenestration of the basilar artery is found in less than 1% of cases. The basilar artery may exist as 2 longitudinal trunks united across the midline. The SCA may be duplicated or absent. The internal auditory artery is most often a branch of the AICA but may arise from the SCA (in up to 25% of cases) or the basilar artery (in less than 20% of cases).
The PCOM may be absent, or the branch representing it may fail to join the posterior cerebral. Fenestration of the basilar artery is found in less than 1% of cases. The basilar artery may exist as 2 longitudinal trunks united across the midline. The SCA may be duplicated or absent. The internal auditory artery is most often a branch of the AICA but may arise from the SCA (in up to 25% of cases) or the basilar artery (in less than 20% of cases).
Pathophysiological Variants
Asymmetry of the circle of Willis results in significant asymmetry of flow and is an important factor in the development of intracranial aneurysms and ischemic stroke.[6] Patients with aneurysms are more likely to have asymmetry or an anomaly of the circle, and the presence of a nonfunctional anterior collateral pathway in the circle of Willis in patients with ICA occlusive disease is strongly associated with ischemic stroke. Uncommonly, persistence of fetal anastomoses is found involving the circle of Willis. These include persistent trigeminal, otic, hypoglossal and proatlantal arteries. These arteries, more or less, unite the internal carotid and vertebrobasilar systems.