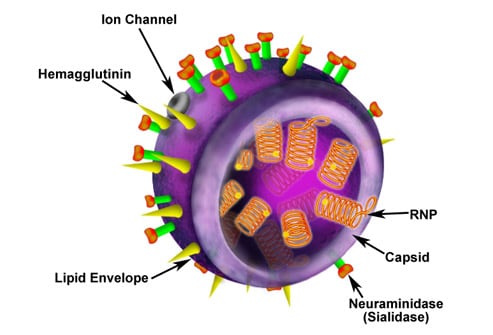
The H1N1 influenza A virus (swine flu) is a novel influenza A virus, more properly termed a new subtype of influenza A (H1N1) that was not previously detected in swine or humans. The H1N1 designation refers to hemagglutinin and neuraminidase -- proteins on the surface of the influenza virus that enable the virus to enter and leave host cells. The conformation of these proteins determines the ability of the virus to infect humans; antibodies to these proteins determine humans' ability to resist the infection. Image courtesy of eMedicine.
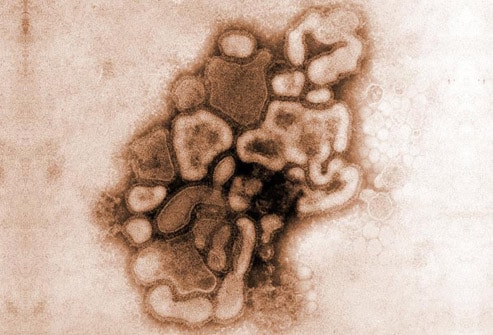
Influenza A strains that can infect mammals (eg, pigs and humans) may undergo genetic reassortment through contact with avian strains. The reassortment of an avian strain with a mammalian strain may produce a chimeric virus that is transmissible between mammals; such mutation products may contain hemagglutinin and/or neuraminidase proteins that are unrecognizable to the immune systems of mammals. This antigenic shift results in a much greater population of susceptible individuals in whom more severe disease is possible. Image courtesy of the CDC/Dr. E. Palmer; R. E. Bates.
Such antigenic shifts as described on the previous slide can cause a pandemic -- only 3 pandemics have occurred in recorded history. The most striking of these pandemics was the 1918 Spanish influenza, which infected one third of the world's population (an estimated 500 million people) and caused approximately 50 million deaths. Despite its name, this pandemic may well have originated in the United States; the first known outbreak occurred at an Army base in Kansas. Image courtesy of the National Library of Medicine.
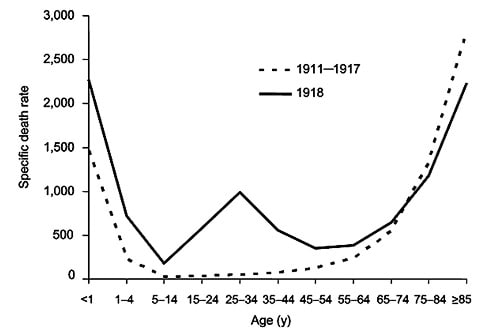
There was a significant difference in age-related mortality distributions between the 1918 pandemic and normal influenza epidemics. This graph shows the deaths per 100,000 persons in each age group in the United States from 1911 to 1917 (dashed line) and in the pandemic year, 1918 (solid line). Image courtesy of the United States Federal Government.
A 1976 outbreak of swine influenza in Fort Dix, New Jersey, involved more than 200 cases, some of them severe, including 1 death. The fear of an influenza pandemic led to a national campaign in the United States designed to immunize nearly the entire population. Approximately 40 million people received the A/NewJersey/1976/H1N1 vaccine before the initiative was halted because of the strong association between the vaccine and Guillain-Barré syndrome. About 500 cases of Guillain-Barré syndrome were reported, with 25 deaths due to associated pulmonary complications. Subsequent influenza vaccines did not have this strong association. Image courtesy of the United States Federal Government.
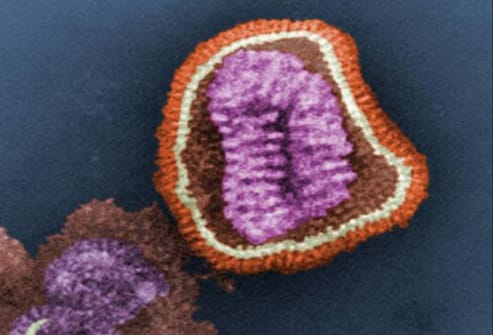
In the current outbreak in the United States, preliminary testing has shown the same genetic pattern in all cases of the virus. The virus is being described as a new subtype of influenza A/H1N1, one that has not previously been detected in either swine or humans. On April 26, 2009, the US Department of Health and Human Services declared a national public health emergency involving swine influenza A, citing its significant potential to affect national security. Image courtesy of the CDC/Dr. Erskine L. Palmer; Dr. M. L. Martin.
In response to the spread of the virus, the World Health Organization raised its pandemic alert level for H1N1 influenza to phase 6 on June 11. Phase 6 is characterized by sustained community-level transmission of the virus in more than 1 World Health Organization region of the world. As of September 30, laboratory-confirmed cases of pandemic influenza H1N1 had been reported in 191 countries and territories worldwide. Image courtesy of the World Health Organization.
Rapid influenza diagnostic tests are commercially available. These tests can provide results within 30 minutes or less for point-of-care decisions. However, they cannot distinguish between seasonal influenza A and H1N1 influenza, nor can they provide information about antiviral drug susceptibility. In addition, a negative result does not rule out H1N1 infection. Confirmation of H1N1 influenza infection requires laboratory testing of a respiratory specimen. Image courtesy of CDC/Greg Sykes, ATCC.
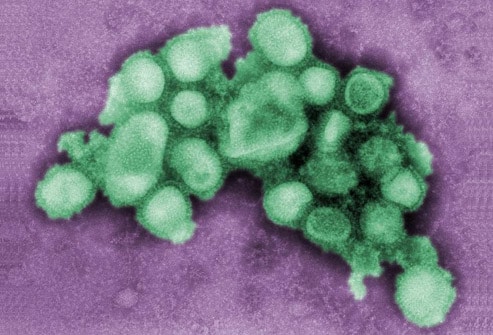
Personnel providing direct patient care for suspected or confirmed cases of H1N1 influenza should wear a fit-tested disposable N95 respirator when entering the patient's room. Healthcare personnel should wash their hands with soap and water or use hand sanitizer immediately after any contact with respiratory secretions. Isolation precautions should be continued for 7 days from symptom onset or until the resolution of symptoms, whichever is longer. Image courtesy of CDC/C. S. Goldsmith and A. Balish.
For confirmed, probable, or suspected cases of influenza A (H1N1) virus infection, healthcare providers should consider antiviral treatment with oseltamivir or zanamivir for 5 days. Hospitalized patients and patients at higher risk for influenza complications should be prioritized. Antiviral treatment is recommended as soon as possible after symptom onset. Antiviral medications should be used during pregnancy only if the potential benefit outweighs the potential risk to the embryo or fetus. Infants younger than 1 year of age who typically have high rates of morbidity and mortality from influenza may benefit from treatment with oseltamivir. Image included with permission and copyrighted by First DataBank, Inc.
In addition to empiric antiviral treatment for confirmed, probable, or suspected cases of influenza A (H1N1), basic supportive therapy, as used in previous pandemics, should be provided and include bed rest, increased fluid consumption, cough suppressants, and antipyretics and analgesics (eg, acetaminophen, nonsteroidal anti-inflammatory drugs) for fever and myalgias. Severe cases may require intravenous hydration and other supportive measures. Image included with permission and copyrighted by First DataBank, Inc.
On September 15, the Food and Drug Administration approved four vaccines for use against the 2009 H1N1 influenza virus. National distribution is expected in October. The vaccine can be given at the same time as seasonal influenza vaccine. Image courtesy of CDC/Judy Schmidt.


ليست هناك تعليقات:
إرسال تعليق